Shares of VIVUS Inc. (VVUS) gained more than 12% to $4.55 in morning trading on Thursday after the U.S. Food and Drug Administration approved the company’s new label impotence drug STENDRA, which cuts in half the time patients need to take the pill before engaging in sexual activity. Previously approved prescribing information recommended that patients with erectile dysfunction [ED] take the drug about 30 minutes before.
“STENDRA is the first FDA-approved ED drug in nearly a decade and offers men and their partners an exciting new option,” Adrian Adams, CEO and President of Auxilium Pharmaceuticals (AUXL) said in a statement.
Approximately 10.2M VVUS shares have already changed hands compared to the stock’s average daily volume of 1,462,930 shares.
On valuation-measures, shares of VIVIUS Inc have a P/E to growth ratio of (0.82), while price-to-sales is 3.09. EPS registers ($1.34). The company has a market cap of $444.77 million and a median Wall Street price target of $4.50 with a high target of $18.00.
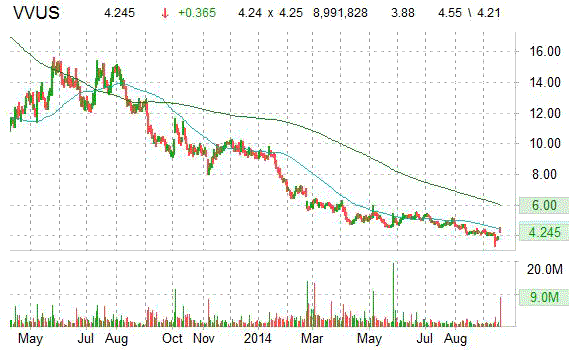
On trading-measure, VVUS has a beta of 1.66 and a short float of 38.20%. In the past 52 weeks, shares of Mountain View, California-based biopharmaceutical company have traded between a low of $3.32 and a high of $11.64 with the 50-day MA and 200-day MA located at $4.32 and $5.02 levels, respectively.
VVUS currently prints a negative one year return of about 58.77% and a year-to-date return of around 54%.
The chart below shows where the equity has traded over the last 52 weeks, with the 50-day and 200-day MAs included.

Leave a Reply