StemCells, Inc (STEM) today announced that is has received authorization from the Swiss regulatory agency for therapeutic products, to initiate a Phase I/II clinical trial in Switzerland of the co.’s proprietary HuCNS-SC(R) product candidate (purified human neural stem cells) in chronic spinal cord injury.
The trial will be conducted in Zurich at the University Hospital Balgrist, University of Zurich, one of the leading medical centers in the world for spinal cord injury and rehabilitation.
“This trial marks a significant broadening of our clinical development program,” said Martin McGlynn, President and CEO of StemCells, Inc. “With this authorization, we will soon have three active clinical trials evaluating our HuCNS-SC cells in conditions affecting both the brain and the spinal cord.”
Shares of STEM are lower on the session by 0.85%, currently trading at $1.16. The stock has been moving largely higher over the past several months as shares have nearly doubled from 52-week lows of $0.75 ticker printed on August 31. More than 2.9 million shares have traded hands so far into the session versus a 12-week day average volume of 1.56 million shares.
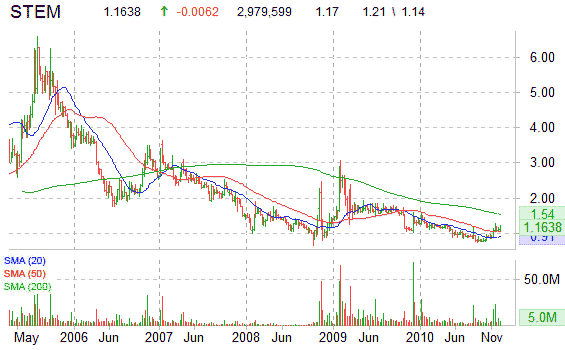
Look for shares of STEM to trade within a new higher trading range with resistance at $1.40.
Disclosure: No Position


Leave a Reply