Dendreon Corp. (DNDN) is squeezing higher this morning, currently trading at $37.00, following a Medicare report of the drugmaker’s prostate cancer drug Provenge, which showed “moderate evidence” that the therapy can improve outcomes.
The release of a draft technology assessment report — posted yesterday on the CMS website — for the Nov. 17 Provenge MEDCAC panel concluded that the three randomized clinical trials of Provenge are “consistent with longer overall survival in patients meeting the FDA-labeled indication.”
Technically speaking, the shares of DNDN have currently added more than 5.8% in electronic trading, the most intraday since Sept. 8, with the shares returning year-to-date 18% as of today’s hod price of $36.72. The ticker continues to maintain its push back up toward intraday highs as buyers try to hold the current move back above the key $35.00 area.
DNDN is currently below its 50-day moving average of $39.16 and below its 200-day moving average of $37.58. The shares recently started pulling back to test the $34.00 mini – support area, and before today’s news it seemed ticker was poised for a retest of the $32.50 support, which acted in early August as a springboard to launch DNDN 10 points higher.
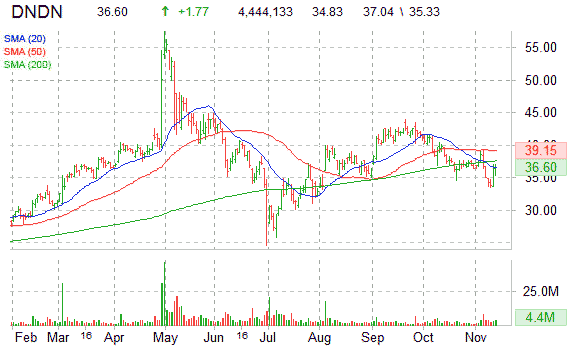
It’s worth pointing out that the ticker’s RSI currently rests at a 48.29 – in relatively oversold terrain – suggesting that more upside may be in the cards.
At last check, Dendreon shares were up more than 5.00%, to $36.60, in midday trading.


Leave a Reply