Shares of Opexa Therapeutics, Inc. (OPXA) spiked more than 70% Wednesday after the cell-therapy firm said that it and the U.S. Food and Drug Administration agreed on the design of a Phase III clinical study to test the company’s experimental multiple sclerosis [MS] drug, Tovaxin. There is no cure for multiple sclerosis, an autoimmune disease that effects an estimated 2.5 million people worldwide.
“We are very pleased with the outcome of our two recent FDA meetings regarding Tovaxin, the first ever personalized T-cell therapy for MS patients, as we believe we now have a well defined path forward for Phase 3 clinical studies,” commented Neil K. Warma, President and CEO of Opexa.
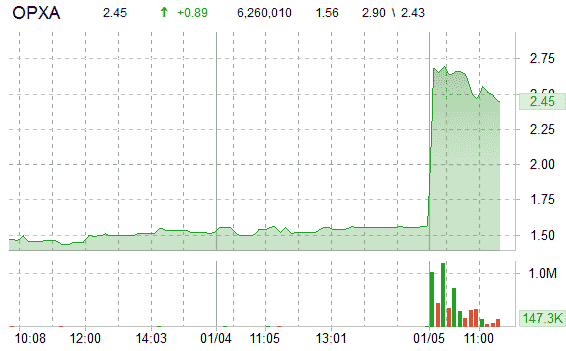
Shares of Opexa, which have risen 8% since it reported quarterly results, are currently up 57.69% at $2.46 per share. Volume has exploded with more than 6 million OPXA shares already trading hands compared to a daily average volume of just 70K.
Today’s trading range for shares of Opexa Therapeutics, Inc has been between $2.43 and $2.90 per share. Over the past one-year, the stock has fallen over 19%. It has a 52-week range of $1.02-$3.07.
The consensus price target of analysts covering the company’s stock is $6.00 per share.
Opexa shares were up nearly $1 to $2.49 before the bell. They closed at $1.56 Tuesday on Nasdaq.
- Bulenox: Get 45% to 91% OFF ... Use Discount Code: UNO
- Risk Our Money Not Yours | Get 50% to 90% OFF ... Use Discount Code: MMBVBKSM
Disclaimer: This page contains affiliate links. If you choose to make a purchase after clicking a link, we may receive a commission at no additional cost to you. Thank you for your support!


Leave a Reply