Shares of medical device maker Mela Sciences, Inc. (MELA) are off sharply in midday trading, on news that the U.S. Food and Drug Administration [FDA] has released documents indicating that the company’s melanoma detection device might be harmful due to the risk of mis-diagnosis.
According to the U.S. health regulators, Mela Science’s MELAFind, a computer-enhanced imaging device that takes high-tech pictures of suspect moles and lesions to assist doctors in the early detection of skin cancer, “has not been studied adequately” and “puts the health of the public at risk”. The FDA also pointed out in its documents numerous problems with company’s study of the device, including a significant lack of data, and urged a new clinical trial.
The documents were released ahead of the FDA advisory panel meeting about the device Thursday.
Mela Sciences rejected the FDA’s view, saying it was “completely without context.”
Technically speaking, shares of MELA are down nearly 29 percent this year, and currently trading below their 50-day moving average of $6.83 and below their 200-day moving average of $7.41. The stock, whose short interest currently accounts for approximately 37% of its 24.11M float, touched a new 52-week low of $2.80 in early trading.
Shares of MELA are lower on Nasdaq by 53.38%, currently trading at $2.97.
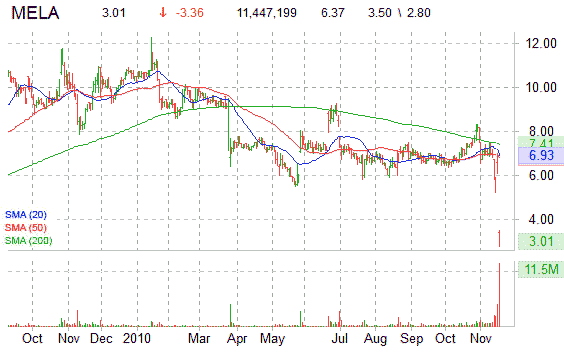
Approximately, 11 million shares have traded hands so far into the session versus 12-week day average volume of 612,000 shares.
Disclaimer: This page contains affiliate links. If you choose to make a purchase after clicking a link, we may receive a commission at no additional cost to you. Thank you for your support!


Leave a Reply