Shares of Avanir Pharmaceuticals Inc. (AVNR) more than doubled in value Monday, rising as much as 120 percent, as news spread that the FDA has approved the co.’s drug Nuedexta, the first treatment for uncontrolled laughing or crying in patients with neurological disorders.
On a conference call with analysts, the Aliso Viejo, Calif. – based company said it plans to launch the new drug in the United States in the first half of FY2011. Bloomberg cited various analysts that said the co. could rake in as much $275 million in sales by FY2013. Meanwhile, peak sales for the new drug are projected to hit $350 million to $500 million.
Also on Monday, the drugmaker’s price target was raised to $15 at Jefferies & Co. by equity analyst Andrew Fein, who in September of this year pegged the drug’s chances of approval at 80 percent or higher.
The small cap stock reached a 52-week high of $5.80 in early trading, and at last check, it was up 108.68% to $5.06, with volume at 41 million vs a 3-m average of 2.2 million.
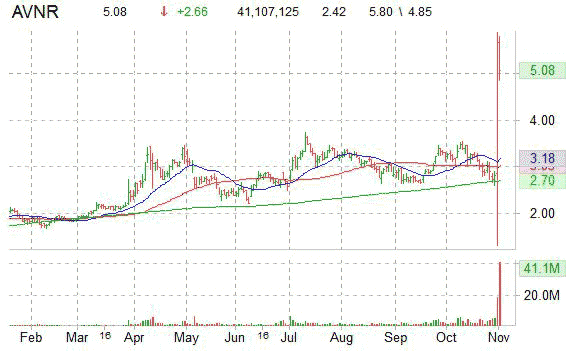
AVNR has a 52-week range of $1.31-$5.80. It is currently trading above its 50-day $3.03 and 200-day $2.70 moving averages.
- Bulenox: Get 45% to 91% OFF ... Use Discount Code: UNO
- Risk Our Money Not Yours | Get 50% to 90% OFF ... Use Discount Code: MMBVBKSM
Disclaimer: This page contains affiliate links. If you choose to make a purchase after clicking a link, we may receive a commission at no additional cost to you. Thank you for your support!


Leave a Reply