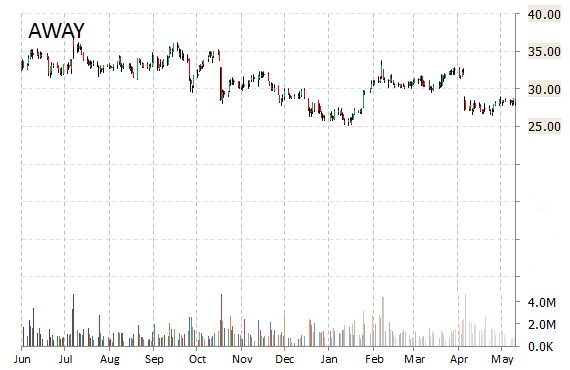
HomeAway, Inc. (AWAY) shares are surging, up 7.60% to $30.20, in early market trading on Monday after Priceline’s (PCLN) Kayak announced an agreement to display HomeAway’s vacation rentals on its site. Integration is expected to be live by the end of the year with nearly 200,000 property listings worldwide – giving Kayak a more diverse set of lodging options for users to search.
“This is another huge step for the industry,” said in a statement Brian Sharples, HomeAway co-founder and CEO. “Vacation rentals will appear more prominently on the leading travel search engine and, more importantly, even more families and groups will realize that they have the option to rent a whole home for their next vacation.”
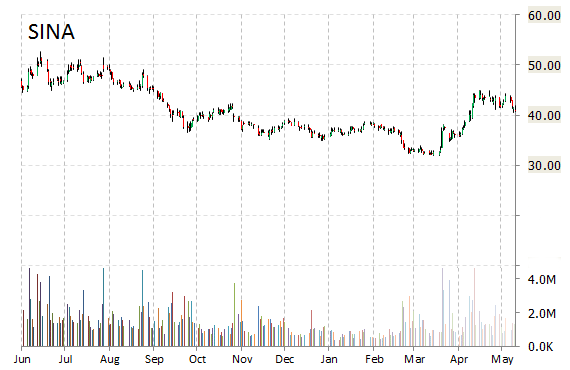
Shares of SINA Corporation (SINA) are skyrocketing by 22% to $49.64 on heavy volume at the start of trading on Monday morning, after the company announced an agreement with Mr. Charles Chao, Chairman of SINA’s board of directors and Chief Executive Officer, for a $456 million cash investment.
About 8.5 million shares of Sina Corporation were traded by 11:20 a.m. ET Monday, above the company’s average trading volume of about 1.5 million shares a day.
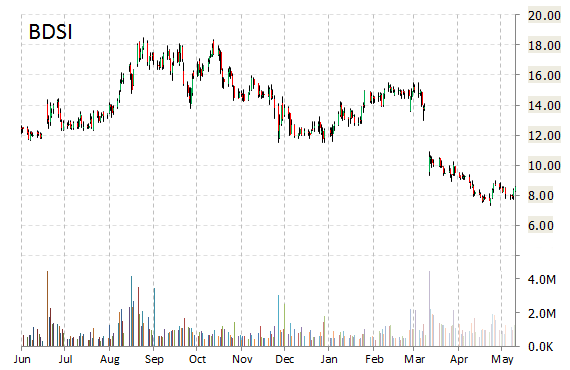
BioDelivery Sciences International, Inc. (BDSI) is plunging 6.70 percent to $7.95 Monday morning. The company announced today that it has secured an additional $20.7 million in gross debt funding from MidCap Financial to bring BDSI’s total outstanding debt with MidCap to $30 million in a single senior secured loan.
Dr. Mark A. Sirgo, President and CEO of BDSI, stated, “This funding bolsters our available capital resources well into 2016 as we continue to make a concentrated effort behind the launch of BUNAVAIL.”
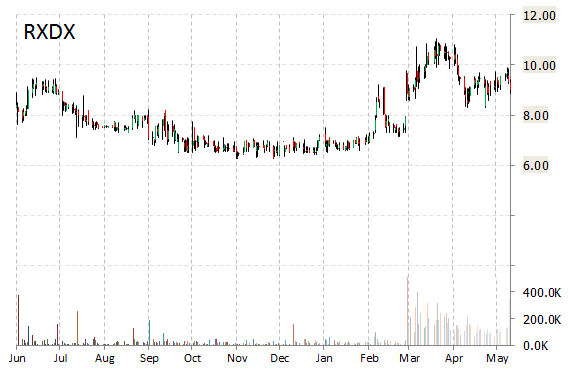
Shares of Ignyta, Inc. (RXDX) are rallying, up nearly 33 percent on Monday morning. The catalyst for the increase is news on Phase 1 clinical trial data for Entrectinib, the company’s proprietary oral tyrosine kinase inhibitor targeting tumors. Ignyta reported 91% response rate in patients meeting expected Phase 2 eligibility criteria.
- Bulenox: Get 45% to 91% OFF ... Use Discount Code: UNO
- Risk Our Money Not Yours | Get 50% to 90% OFF ... Use Discount Code: MMBVBKSM
Disclaimer: This page contains affiliate links. If you choose to make a purchase after clicking a link, we may receive a commission at no additional cost to you. Thank you for your support!


Leave a Reply