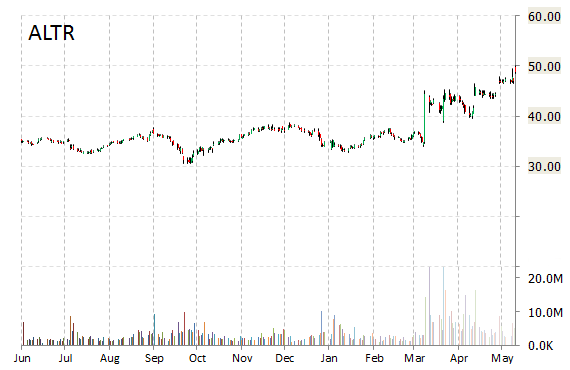
Shares of Altera Corp (ALTR) rose 6% to $51.87 this morning on news chipmaker giant Intel (INTL) has agreed to buy the company for $54 a share, or about $16.7 billion, in an all-cash deal.
Intel said that it plans to fund the acquisition, which is expected to close within six to nine months, with a combination of cash from the balance sheet as well as debt. Intel expects the deal to be accretive to its non-GAAP EPS and free cash flow in the first year after close.
Both boards have approved the transaction.
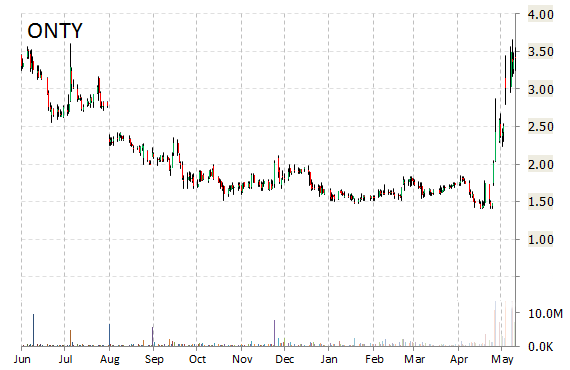
Shares of Oncothyreon Inc (ONTY) were up more than 25% this morning after the presentation of positive data from the company’s ongoing trials of ONT-380, an orally active, reversible and selective small-molecule HER2 inhibitor for the treatment of breast cancer, at the American Society of Clinical Oncology [ASCO] 2015 Annual Meeting.
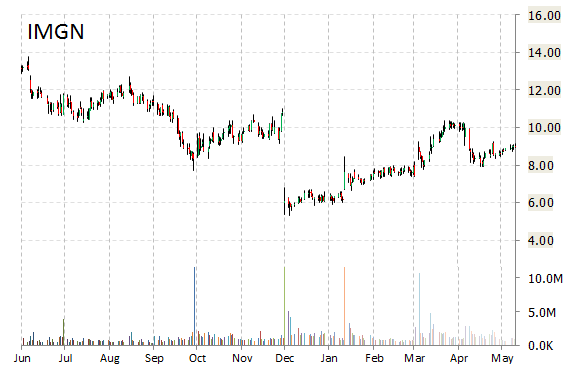
Shares of ImmunoGen, Inc. (IMGN) were up nearly 26% in pre-market hours after the company announced the presentation at the 2015 ASCO Annual Meeting of the first clinical findings in a disease-specific patient population with the company’s unique, FRα-targeting ADC, mirvetuximab soravtansine (abstract #5518). ImmunoGen said its Mirvetuximab Soravtansine (IMGN853) demonstrates notable single agent activity for patients with Platinum-Resistant ovarian cancer.
“These initial clinical findings with mirvetuximab soravtansine in the treatment of patients with FRα-positive platinum-resistant ovarian cancer are highly encouraging,” commented Dr. Kathleen Moore, Mai Eager Anderson Chair of Cancer Clinical Trials, Stephenson Cancer Center, University of Oklahoma HSC. “There is a significant need for new therapies for patients with ovarian cancer, including platinum-resistant disease.”
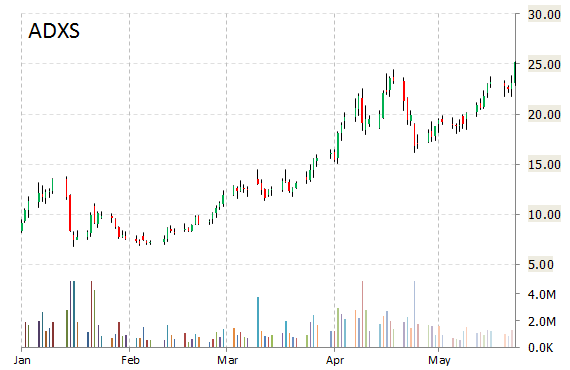
Shares of Advaxis, Inc. (ADXS) are higher by 3.30% to $25.99 in pre-market trading on Monday. The company announced FDA clearance of investigational new drug application for phase 2 study of ADXS-HPV.
“We are very pleased that the IND for this Phase 2 study has been cleared by the FDA,” said in a statement Rich Levy, MD, Chief Drug Development Officer at Incyte. “Epacadostat is currently in multiple combination proof-of-concept trials with immune checkpoint inhibitors, and this new study may provide us with important translational data for epacadostat in combination with an immunotherapeutic vaccine.”
- Bulenox: Get 45% to 91% OFF ... Use Discount Code: UNO
- Risk Our Money Not Yours | Get 50% to 90% OFF ... Use Discount Code: MMBVBKSM
Disclaimer: This page contains affiliate links. If you choose to make a purchase after clicking a link, we may receive a commission at no additional cost to you. Thank you for your support!


Leave a Reply