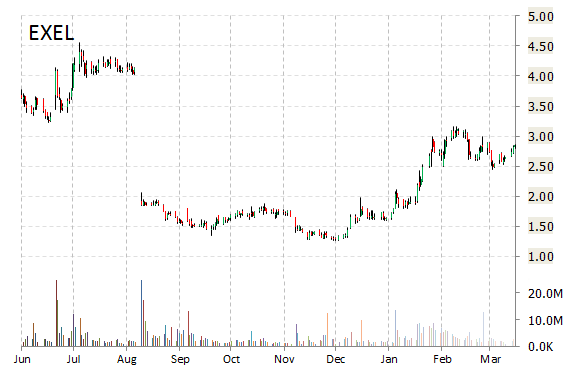
Shares of Exelixis, Inc. (EXEL) are higher by nearly 11% in early morning trading on Thursday, as the stock continues to see gains following news that the FDA has granted Fast Track designation to cabozantinib for treatment of patients with advanced renal cell carcinoma (RCC) who have received one prior therapy.
Cabozantinib is Exelixis’ lead compound and inhibits the activity of multiple tyrosine kinases including MET, VEGFRs and RET. The FDA created the Fast Track process to facilitate the development and expedite the review of drugs to treat serious diseases and address unmet medical needs.
EXEL shares recently gained $0.29 to $3.14. The stock is down more than 21% year-over-year and has gained roughly 98% year-to-date. In the past 52 weeks, shares of South San Francisco, California-based biopharmaceutical company have traded between a low of $1.26 and a high of $4.55.
Exelixis, Inc. closed Wednesday at $2.85. The name has a total market cap of $615.11 million.
- Bulenox: Get 45% to 91% OFF ... Use Discount Code: UNO
- Risk Our Money Not Yours | Get 50% to 90% OFF ... Use Discount Code: MMBVBKSM
Disclaimer: This page contains affiliate links. If you choose to make a purchase after clicking a link, we may receive a commission at no additional cost to you. Thank you for your support!

Leave a Reply