Shares of Ariad Pharmaceuticals Inc. (ARIA) are up more than 5% in pre-market trading after the company this morning announced that Health Canada has approved the use of Iclusig in Canada for the treatment of adult patients with all phases of chronic myeloid leukemia (CML) or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) for whom other tyrosine kinase inhibitor (TKI) therapy is not appropriate, including CML or Ph+ ALL that is T315I mutation positive, or where there is prior TKI resistance or intolerance.
“Patients with CML or Ph+ ALL can become resistant to their therapies over time,” said in a statement Professor Jeffrey Lipton, Ph.D., M.D., staff physician at The Princess Margaret Cancer Centre and one of the PACE trial investigators. “Iclusig will be a valuable new therapeutic option in Canada for patients with refractory CML and Ph+ ALL, where we have a proven unmet medical need.”
Last month, Ariad received approval of Iclusig (Ponatinib) in Israel.
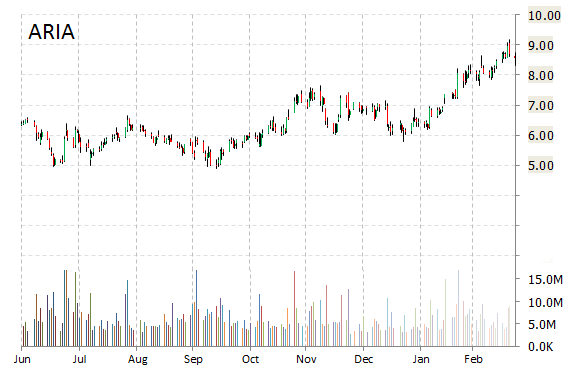
ARIA shares recently gained $0.42 to $8.60. In the past 52 weeks, shares of Cambridge, Massachusetts-based firm have traded between a low of $4.90 and a high of $9.19. Shares are up 1.49% year-over-year and 19.07% year-to-date.
- Bulenox: Get 45% to 91% OFF ... Use Discount Code: UNO
- Risk Our Money Not Yours | Get 50% to 90% OFF ... Use Discount Code: MMBVBKSM
Disclaimer: This page contains affiliate links. If you choose to make a purchase after clicking a link, we may receive a commission at no additional cost to you. Thank you for your support!


Leave a Reply