Shares of Chimerix, Inc. (CMRX) gained more than 8% to $32.37 in morning trading today after the company announced that the FDA approved the company’s drug brincidofovir for potential use in patients with Ebola.
“Chimerix is committed to working with global health organizations and government agencies in the fight against the Ebola virus outbreak,” M. Michelle Berrey, President and Chief Executive Officer of Chimerix said in a statement. The company also said it is, “working closely with the FDA to finalize a clinical trial protocol early this week to assess the safety, tolerability, and efficacy of brincidofovir in patients who are confirmed to have an infection with the Ebola virus.”
Approximately 1,041,884 CMRX shares have already changed hands, compared to the stock’s average daily volume of 354,547 shares.
On valuation-measures, shares of Chimerix, Inc. have a price-to-book and price-to-sale ratio of 5.58 and 307.22, respectively. EPS registers at ($-1.35). The company has a market cap of $1.15B and a median Wall Street price target of $33.00 with a high target of $37.00.
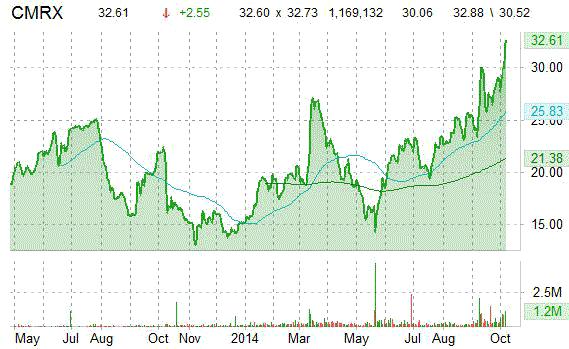
On trading-measure, CMRX has a beta of 1.02 and a short float of 6.75%. In the past 52 weeks, shares of Durham, North Carolina-based biopharma company have traded between a low of $12.96 and a high of $32.86 with the 50-day MA and 200-day MA located at $26.58 and $22.46 levels, respectively.
CMRX currently prints a one year return of about 37.45% and a year-to-date return of around 98.94%.
The chart below shows where the equity has traded over the last 52 weeks, with the 50-day and 200-day MAs included.
Disclaimer: This page contains affiliate links. If you choose to make a purchase after clicking a link, we may receive a commission at no additional cost to you. Thank you for your support!

Leave a Reply